
These resins which use in the water softening system can generate and regenerated by NaCl or KCl salt. Hard water interferes cleaning process of soap.Ĭommercial and residential water-softening units use ion-exchange resins to remove calcium, magnesium, and other metal cations from hard water. The resulting soft water does not build up insoluble scales or precipitates in household and industrial equipment and pipes. Water softening is the process of removal of calcium, magnesium, and other metal cations from hard water. These industrial chemicals are used widely in the Soda-ash industry for manufacturing glass, dyes, and many other domestic and commercial products.
#NACL LATTICE STRUCTURE SKIN#
Paper, rubber, textiles, pharmaceuticals, skin care, water softening, and tanning industries are other chief consumers of sodium chloride salt. Sodium chloride usesĪnnually, a million tonnes of sodium chloride salt is used for the production of many sodium and chlorine compounds. Therefore, when NaCl is mixed with water, the solution is neither basic nor acidic. NaCl is formed from the neutralization reaction of a strong acid (HCl) and a strong base (NaOH). The pH of aqueous sodium chloride solution ≈7. To make a saturated solution, we use 36 g of NaCl in 100 g of water at 293 K. Therefore, a highly polar solvent like water is used to make the NaCl solution. The attraction between the Na + and Cl − ions in the solid is very strong. It appears to be a white crystalline solid in pure form and dissolved in water to form a colorless solution. The seawater contains approximately 2.7-2.9% NaCl which is the major factor in the saline nature of seawater. In solid NaCl, each Na + ion is surrounded by six Cl − ions, or each Cl − ion is surrounded by six Na + ions. Some common properties of NaCl are given below the table, IUPAC nameĬrystallographic studies show that there is no discrete NaCl molecule.
#NACL LATTICE STRUCTURE FREE#
The aqueous solution of sodium chloride is a good conductor of electricity due to the free movement of Na + and Cl − ions. It is soluble in water, ammonia, methanol, etc. NaCl provides flavors in food and is used as a binder and stabilizer. It is named common salt, rock salt, table salt, regular salt, sea salt, halite, and saline. Sodium chloride is a white crystalline solid with a molar mass of 58.443 g.

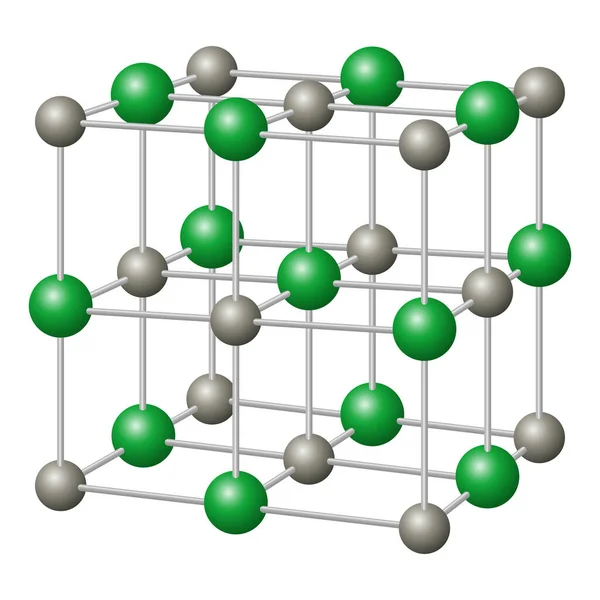
The packing of two ions is repeated throughout in a non-ending pattern. The above picture shows that Cl − ions are located in the corner of the cube and center of the six faces while Na + ions are located halfway between two chloride ionsĮach sodium ion in NaCl crystal is shared equally by six chloride ions and each chloride ion by six sodium ions. The two ions Na + and Cl − then build up a packed type ionic crystal structure. The arrangement of Na + and Cl − ions in NaCl crystal is given below the picture, NaCl crystal belongs to a face-centered cubic crystal lattice. Rock salt or sodium chloride is an ionic compound that forms a white crystalline solid. Naturally, it occurs in seawater and mineral halite. It is an ionic compound formed by the ionic bonding of sodium and chlorine atoms. Sodium chloride dissolves in water to form a saline solution with pH ≈7. It is used mainly for the production of sodium hydroxide (NaOH), sodium carbonate (Na 2CO 3), sodium sulfate (Na 2SO 4), hydrogen, chlorine, hydrochloric acid, etc. NaCl is the starting material for the production of various sodium and chlorine compounds. The use of sodium chloride in the chemical industry is simply numerous. The two oppositely ions Na + and Cl − are held together by ionic bonding to form crystalline sodium chloride salt. Similarly, the chlorine atom gains one electron to form a uni negative chloride ion.

Each sodium atom loses one electron to form a unipositive sodium ion (Na +).


 0 kommentar(er)
0 kommentar(er)
